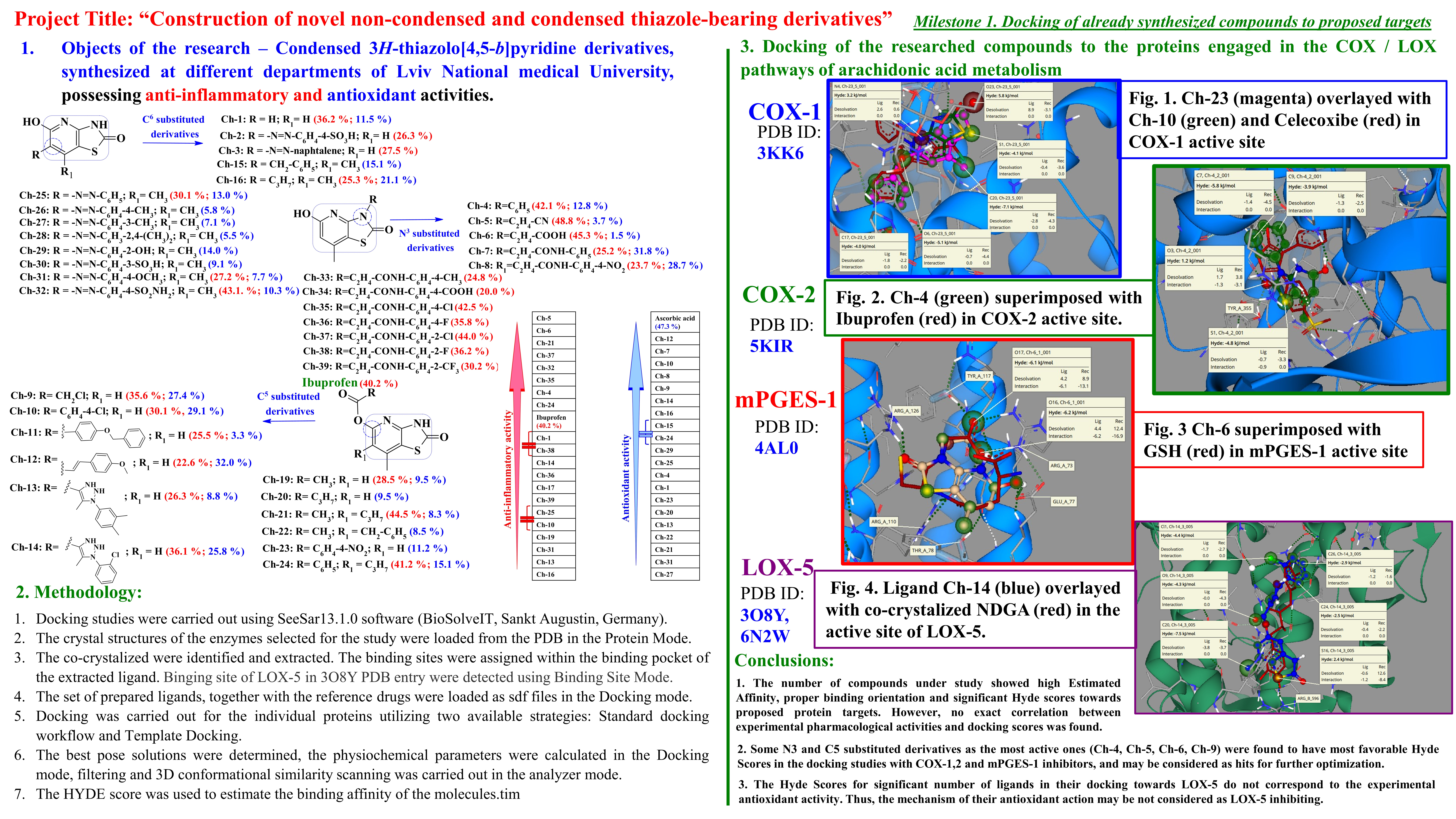
Objects of the research: The library of 37 compounds – condensed 3H-thiazolo[4,5-b]pyridine derivatives, synthesized at different departments of Lviv National Medical University, possessing anti-inflammatory and antioxidant effects, was composed. Docking studies, filtering and poses grouping of the researched compounds towards the proteins engaged in the COX / LOX pathways of arachidonic acid metabolism (COX-1, COX-2; mPGES-1; LOX-5) was carried out utilizing two available strategies: Standard docking workflow and Docking with Template. Three compounds showed higher Hyde Scores and Estimated affinity as compared with COX-1. In both docking procedures (with and without a Template) for the ligands their Estimated Affinity towards COX-2 was significantly lower than that one for Ibuprofen despite they were able to achieve proper orientations. We attended to explore the ability of the ligands competitively interact and replace the Glutathione in the binding site of mPGES-1.
After 3 months, Olena has achieved the following milestones:
- It was found out that Ch-6 was more active than GSH according to docking outcome. Ch-6 perfectly overlayed with GHS, its Estimated affinity was lower, but it had more favorable Hyde scores than GSH. In docking with LOX-5 all ligands have the proper orientation in the binding site, while the experimental activity does not correlate directly with the Estimated affinity. No exact correlation between experimental pharmacological activities and docking scores was found. Compounds possessing the highest anti-inflammatory activity are N3 substituted derivatives, they may be considered as hits for further optimization. The highest Estimated Affinity towards LOX-5 was identified for C5 substituted derivatives. But seems that the mechanism of their antioxidant action may be not considered as LOX-5 inhibition.
- Benchmarks: To make careful analysis of those compounds which possessed considerable activity but were not recognized as proper ligands in any of the docking studies. To analyze the nature of substituents in thiazolopyridine scaffold for such compounds and make conclusions concerning structural patterns which may prevent their proper Estimated affinity towards proposed proteins. To perform in silico evaluation of physicochemical drug-like properties and ADMET-profiles of compounds under study in order to reveal their possible bioavailability. To compare ADMET-properties with the docking results. To perform docking studies of most active compounds with another pro-inflammation biological targets namely LTA4H and soluble Soluble epoxide hydrolase (sEH) in order to disclose the anti-inflammatory action mechanism. These proteins have been recently reported as potential anti-inflammatory targets. A few sets of compounds were prepared for their antitumor activity virtual screening.
- Scaffold Hopper and Motif Matcher Modules of infiniSee allowed to identificate 5 scaffolds with the pharmacophoric similarity of 6.5 % - 32.0 % and 7 skeletons with the similarity of 2.9 % - 57.1 % by filtering compounds according to RO3 criteria for the set of compounds under study.