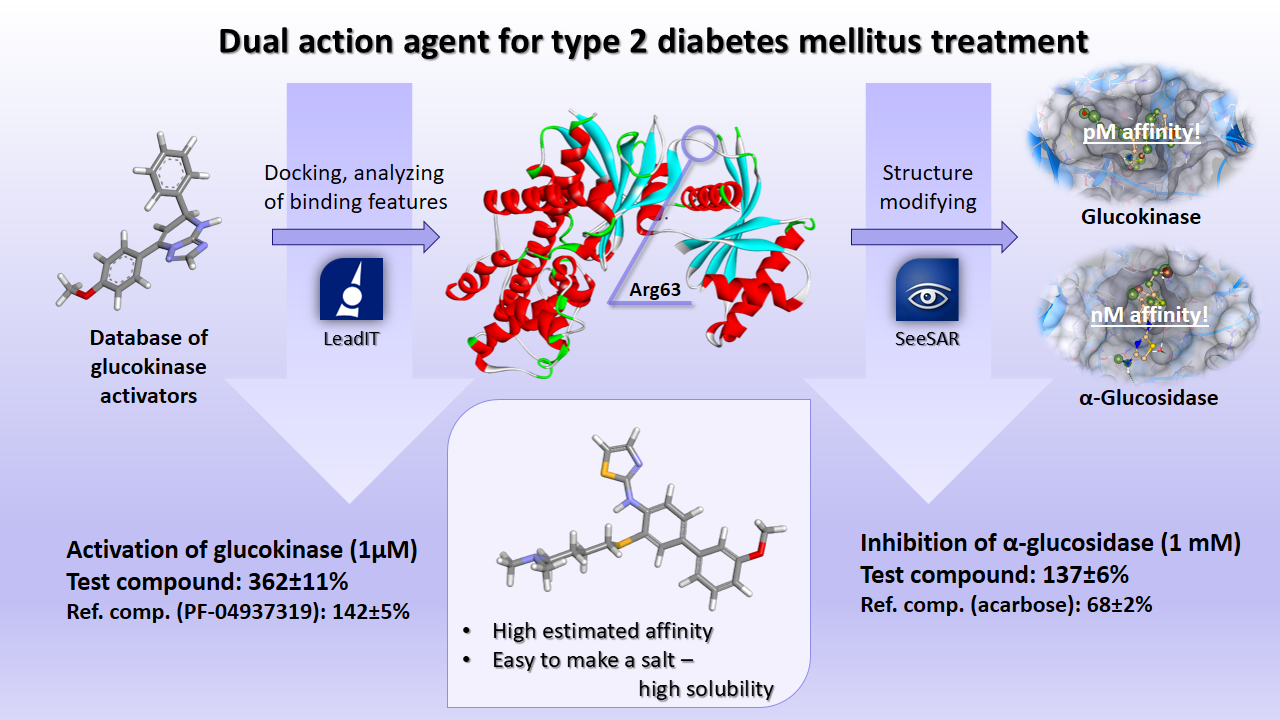
For 12 months of fruitful work we have been able to find out the key factor causing the pharmacological activity of the ligand, to design a molecule with potential activity for both glucokinase and a-glucosidase, and to synthesize and test this compound. In the course of our work, the two programs have become indispensable helpers: LeadIT has helped us to reveal the regularity in the ligand's manifestation of biological activity, and with the help of SeeSAR we constructed dual action agent, which was confirmed by biological tests.
After 1 year, Nadezhda has achieved the following goals:
- To understand the key ligand-receptor interactions that cause pharmacological activity. This is extremely important in the development of the glucokinase activator, since the regulation of the enzyme is carried out through the allosteric center. We used LeadIT soft for docking more than 50 known glucokinase activators, as well as previously developed by us compounds with different activity against glucokinase, and compared the set of amino acids to which each of the compounds binds using 2D diagrams. This allowed us to find out that the necessary condition for an active compound is its ability to interact with Arg63 of the binding site. It was the key data for our further research.
- To create a molecule with potential activity for both glucokinase and a-glucosidase. No doubt the most effective tool for implementing this goal is the SeeSAR program. We sequentially built the backbone of the molecule, using two proteins as a reference point, and then modified the resulting scaffold to achieve the best affinity indices in both cases. So we were able to achieve pM affinity for glucokinase and nM one for a-glucosidase.
- To confirm results in practice. We synthesized 20 molecules with minor modifications of the leader compound. The assembly of the created scaffold was more complicated than we expected, and was associated with the development of new synthetic approaches. All obtained compounds were sent for biological studies. Unfortunately, at the time of writing this report, we received data only for one of the substances. The test substance showed activity superior to the reference compound in the case of glucokinase and statistically significant inhibits the a-glucosidase.