Usually the process of inflammation is accompanied by neurodegenerative diseases. It may be either a nonspecific response of cells to the nervous system disease or it causes the disease. It is known that if patients take non-steroidal anti-inflammatory drugs, they are unlikely to develop these diseases. Some derivatives of the (-)-cytisine are known for high neuropharmacological activity. These compounds possess pronounced mnestic activity, comparable with the activity of piracetam, and also demonstrate anti-inflammatory activity. Within this context, some of (-)-cytosine derivatives can be considered as substances with two therapeutic indications: anti-inflammatory and neuropharmacological.
In the first case the COX-1/2 receptors were considered as biological targets responsible for the inflammatory process. Secondly we examined ionotropic glutamate receptors, involved in learning and memory formation.
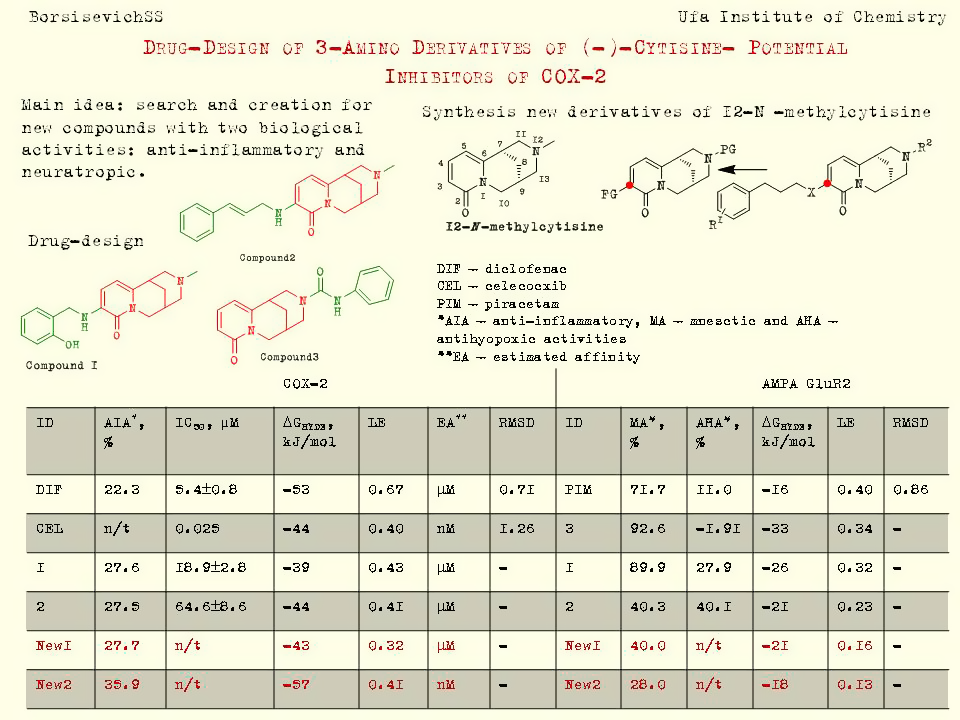
According to biological experiments and in silico studies we selected two lead-compounds among these derivatives of 12-N-methylcytisine (see figure). These compounds have demonstrated ability to inhibit the oedema comparable with our reference drug diclofenac in carrageenan-induced model of inflammatory in vivo and had a moderate ability to inhibit COX-2 (in vitro test).
According to the molecular docking results we suppose that the mnestic activity of derivatives of 12-N-methylcytisine can be explained by its effect on the work of AMPA and KA-receptors.
The molecular structures of these compounds were modified saving the molecular core (cytosine fragment). We pursued two goals: on the one hand, to increase the affinity for the tyrosine site of COX-2, on the other hand save the high value of mnestic activity.
Based on the calculation results, we synthesized two compounds for the in vivo test. New derivatives of (-)-cytisine demonstrated pronounced anti-inflammatory activity exceeding the activity of diclofenac and had a moderate mnestic activity (see figure). Theoretical calculations correlate with the results of biological tests.
However, all of the above is just the first step towards creating new compounds with two biological activities. A number of additional studies are needed.
After 1 year, Sophia has achieved the following goals:
- We used a bioisosterism strategy for rational design of new derivatives of (-)-cytisine and created a database of new compounds. We have chosen biological targets and studied their activity. We estimated ADME parameters of new compounds and have performed the docking process into the tyrosine active site of COX-2 and binding sites of AMPA and KA-receptors.
- Accordind to the calculation results we selected lead-compounds and synthesized new compounds with anti-inflammatory activity. They have demonstrated ability to inhibit the oedema comparable with diclofenac in carrageenan-induced model of inflammation in vivo.
- We suppose that mnestic activities of 12-N-methylcytisine derivatives can be explained by their effect on the work of AMPA and KA-receptors.