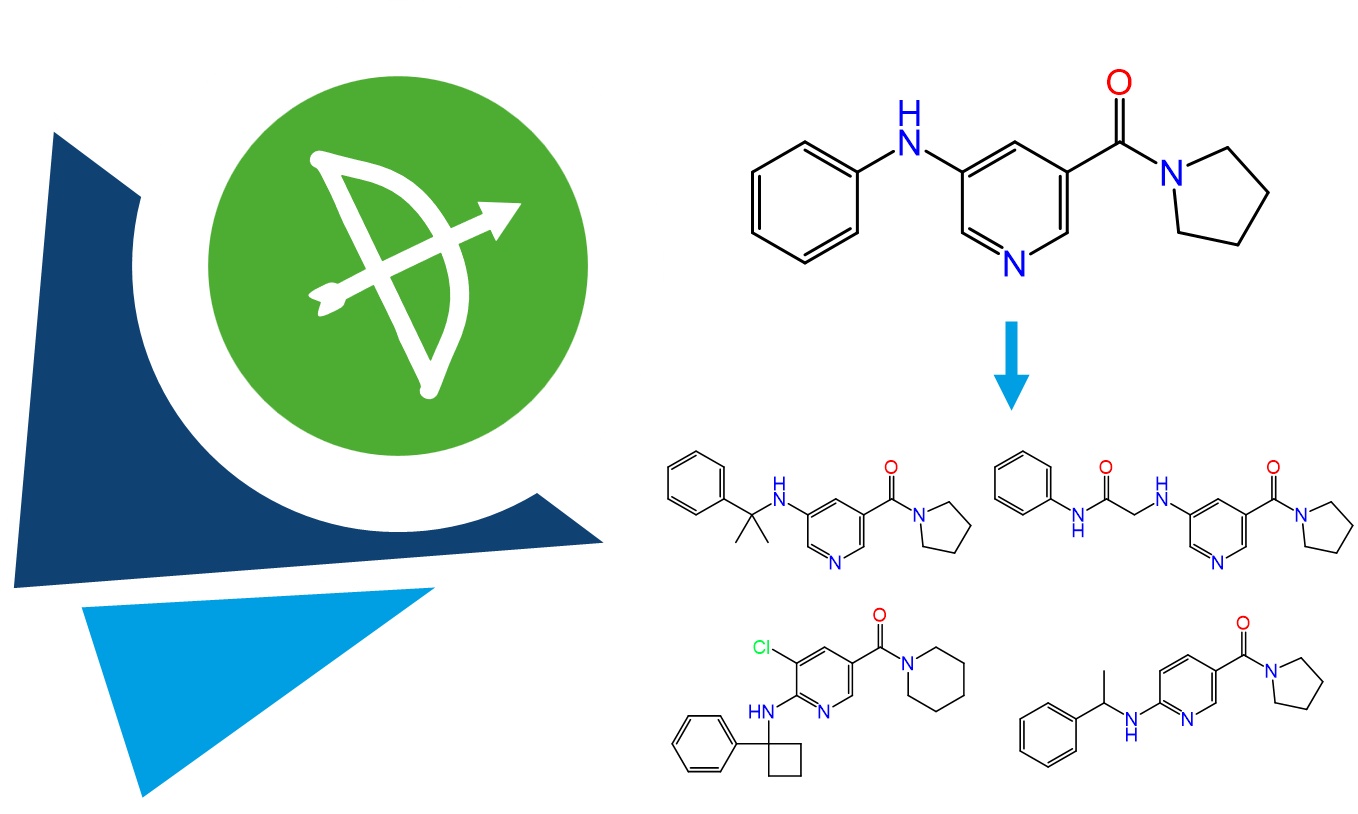
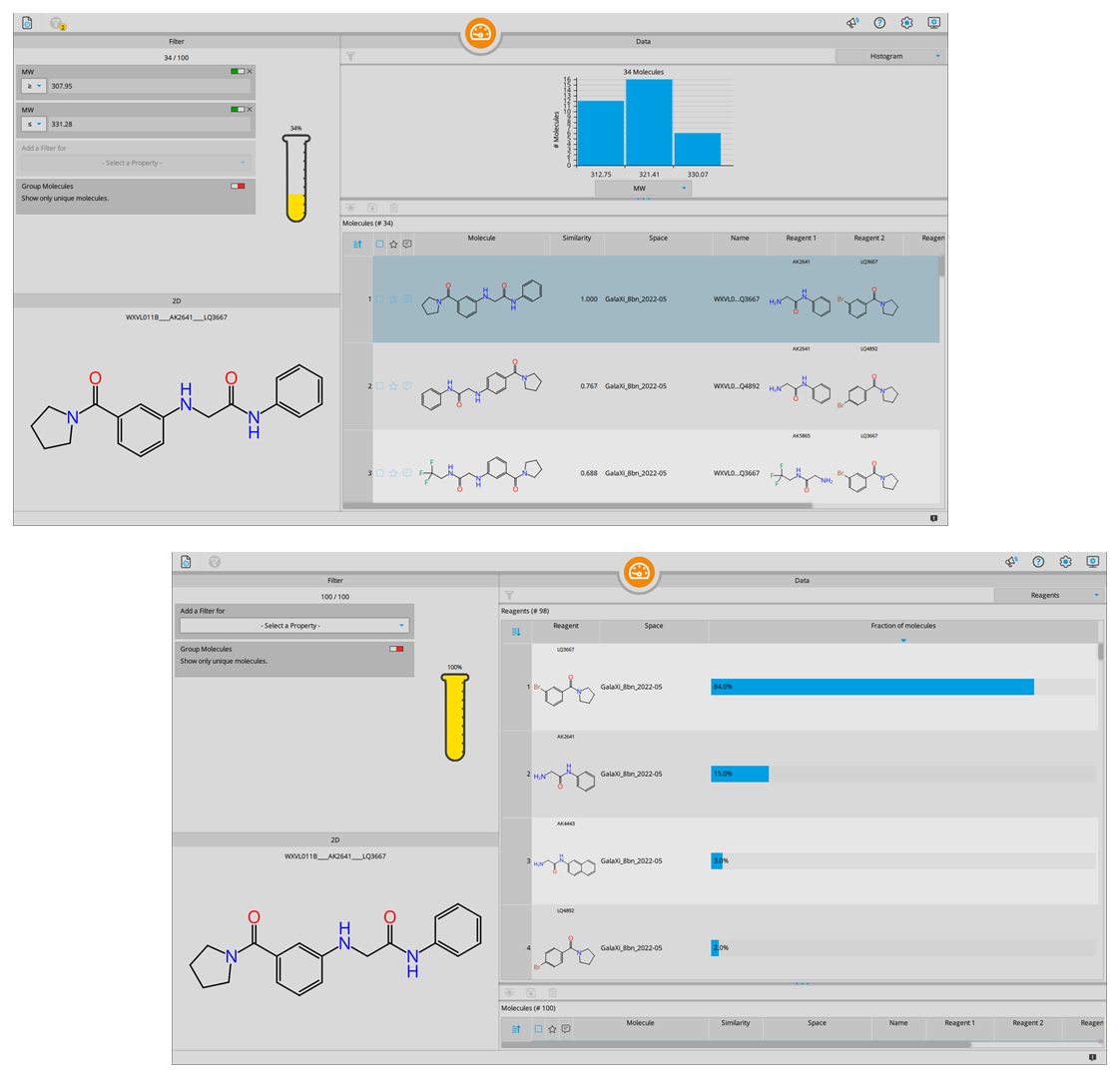
The Analyzer Mode received a visual update as well as several innovations to improve the compound management.
Visual histograms now show the distribution of calculated parameters which can subsequently be used for filtering of the data set.
The novel reagent analysis displays the distribution of individual building blocks used in the combinatorial generation of your retrieved results. This feature is especially suitable for follow-up of relevant scaffolds and for clustering of results.
- Rule of Five parameters: Molecular weight, logP, number of H-bond donors and acceptors, rotatable bonds
- Total polar surface area (TPSA)
- Number of heavy and aromatic atoms, (aromatic) ring systems, halogens
- Maximum ring size
- Number of stereo centers and stereo bonds
- Total charge of entry molecule
Further, co-users of Optibrium software can calculate a great variety of parameters (e.g. 2C9 pK
i, 2D6 affinity, blood-brain-barrier categories, own models, etc.) for molecule sets within the Analyzer Mode and use them for filtering according to their needs.