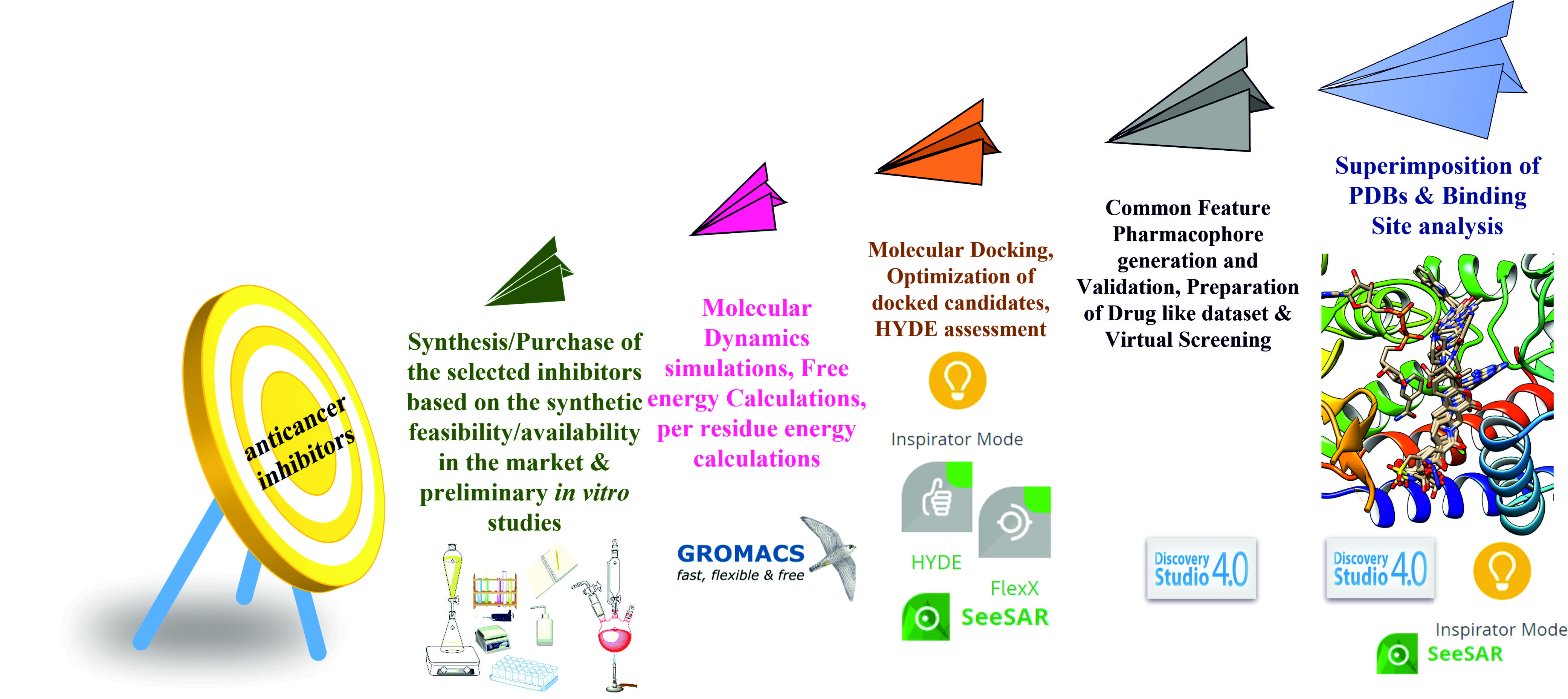
The methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) enzyme is identified as one of the new and attractive anticancer drug targets. As per recent studies, MTHFD2 has an allosteric binding site that coexists with the substrate analog. They confirm that the binding of the inhibitors in the allosteric site leads to disruption in the enzyme mechanism. Also, the allosteric site inhibition is gaining importance owing to the increase in the selectivity of the inhibitors, and reduction in the emergence of drug resistance. Given this importance, our work will present the sheer use of various computational techniques for the identification and validation of the hit molecules. In the current study, we will report the application of various structure-based in silico methods available in Discovery Studio, BioSolveIT, and GROMACS software. To the best of our knowledge, this study will be the first report on the identification of MTHFD2 inhibitors using various computational tools. The reported crystallized complexes of MTHFD2 enzymes were divided into two groups based on their site of inhibition (allosteric and main active site). The complexes from the respective groups were superimposed to generate two coordinate files which were further subjected to pharmacophore modeling. Pharmacophore modeling and validation process were performed on the active site inhibitors also to check the specificity of the models constructed from the allosteric binding site. To conduct the virtual screening (VS) process, various natural product (NP) databases were selected. Finally, a dataset of 236,561 molecule was prepared to conduct VS process over the representative pharmacophores. After screening we retrieved unique 494 candidates wchich were further subjected to molecular docking, HYDE assessment, and the shortlisted candidates were prepared as per Lipinski’s rule of five and Veber's rule. From docking, and HYDE assessment, we obtain 72 molecules, which were further shortlisted to 63 based on the druglikeness studies. On conducting ADMET studies, only 20 molecules were selected. Only seven were selected based on the presence of interaction with important amino acids. These seven candidates were subjected to molecular dynamics simulations. In simulations, we examined RMSD, RMSF, Rgyr, and H-bonds plots for all the docked systems along with reference and apo-protein. From the outcome, we can conclude that all the shortlisted docked candidates displayed higher stability than the selected reference and apo-protein. From binding free energy calculations, we observed that all the compounds showed higher energy than the selected reference. The synthesis of these compounds is under process, and after synthesis, they will be evaluated for their anticancer studies.
After 1 year, Anu has achieved the following goals:
- The collected protein-ligand complexes of MTHFD2 crystallized in the allosteric and actual sites were superimposed. These two superimposed files were further subjected to pharmacophore generation. From the superimposed active and allosteric coordinate file, four and five feature models were constructed respectively. To check the specificity of the allosteric site models, pharmacophore modeling was conducted on the active site too. Some common features like hydrogen-bond acceptor, hydrophobic-aromatic, and ring-aromatic were observed. However, unique features like the hydrophobic-aliphatic, hydrogen-bond donor, and hydrophobic were observed in the allosteric model explaining the specificity. Further, validation outcomes also support the specificity of the models. For the virtual screening process, the natural product (NP) database of 236,561 molecules was selected.
- Based on the outcome of the validation process of the pharmacophore modeling, all the models generated from the allosteric inhibitors were selected to perform the VS using the NP databases. All the retrieved candidates were compiled in one coordinate file and prepared to remove the structural duplicates. After that, 494 screened candidates were selected to conduct molecular docking using the FleXX module. Out of 494, 339 molecules were docked in the allosteric binding site. All the docked candidates were further subjected to HYDE assessment to check their ligand efficiency, and only 72 displayed good ligand efficiency. These 72 molecules were subjected to druglikeness and pharmacokinetic studies. Thus, we retrieved 63 molecules from druglikeness studies, which were further shortlisted to 20 based on the acceptable ADMET parameters. Owing to the presence of crucial interaction, only seven candidates were shortlisted for conducting the simulation studies.
- All the selected candidates will be subjected to molecular dynamics simulations and free energy calculations. From the simulation output, we study the protein-ligand RMSD, RMSF, Rgyr, H-bond, and free energy calculations. Based on the stable trajectory retrieved after 200 ns, binding free energy as computed. From the outcome of the selected paramenters like RMSD, RMSF, Rgyr, and H-bond, we can conclude that all the shortlisted docked candidates displayed higher stability than the selected reference and apo-protein. Also, from the binding free energy calculations, we observed that all the compounds showed higher energy than the selected reference. Further, the synthesis of these compounds is under process and after synthesis, they will be evaluated for their anticancer studies.