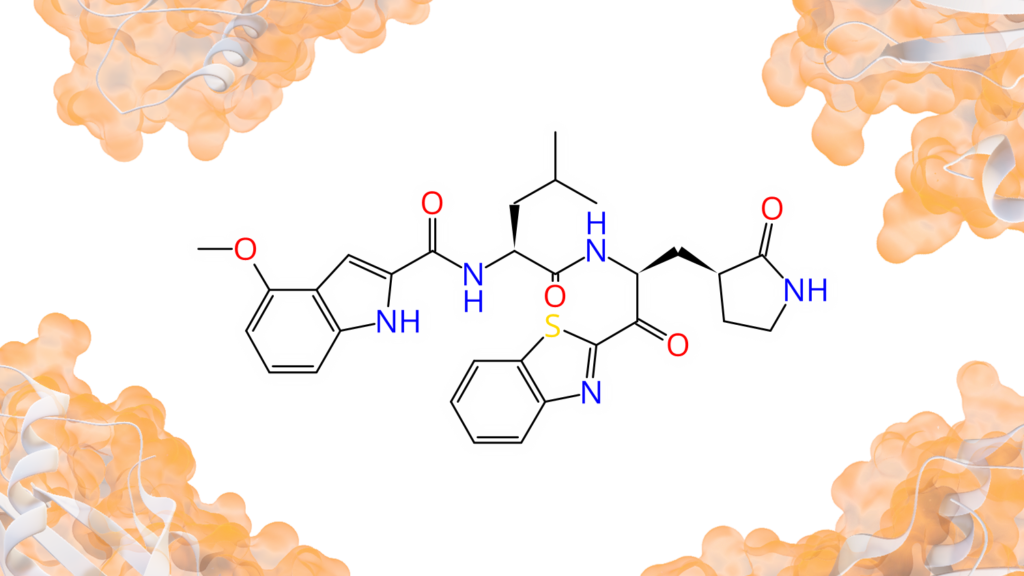
Peter Ertl extracted bioactive ring systems and their respective targets from a billion of drug-like molecules resulting in the creation of a ring Chemical Space with almost 40,000 molecular scaffolds.
Rings represent privileged motives in medicinal chemistry as they define the shape of the molecule, are responsible for the orientation of attached groups and often contribute to the binding affinity on their own. Yet sometimes ring replacement (also described as scaffold replacement) is required due to promiscuity of the system, unfavored physicochemical properties, toxicity issues, or intellectual property claims.
BioSolveIT software tackles this problem with the scaffold replacement tool
ReCore and
infiniSee that performs fuzzy pharmacophore search for distant neighbors to a query molecule.